
It is interesting to repeat this exercise for the silver halides, which have either the NaCl structure (AgF, AgCl, AgBr) or zincblende structure (AgI). The errors in this case are only about 1% of E L. The table below shows results of more detailed lattice energy calculations for ionic fluorides in which the van der Waals term is explicitly included. We can do better by explicitly including the short-range van der Waals attractive energy between ions. The two errors partially compensate, so the overall error in the calculation is small. If we underestimate the attractive energy of the crystal lattice, the energy minimization criterion ensures that the repulsion energy is underestimated as well. This is because we used energy minimization to obtain the repulsion energy in the Born-Mayer equation. The result is promising because we neglected the van der Waals term.īut.how did we get away with neglecting the van der Waals term? Here we have to subtract 2RT to convert our cycle of energies to a cycle of enthalpies, because we are compressing two moles of gas in making NaCl(s) and PΔV = ΔnRT, where Δn = -2.Įxperimentally ΔH f for NaCl is -411 kJ/molīecause all the other numbers in the cycle are known accurately, the error in our calculation is only about 15 kJ (about 2% of E L). There are several different equations, of various degrees of complication, for calculating lattice energy in this way.\)
#Calculate lattice energy of nacl how to#
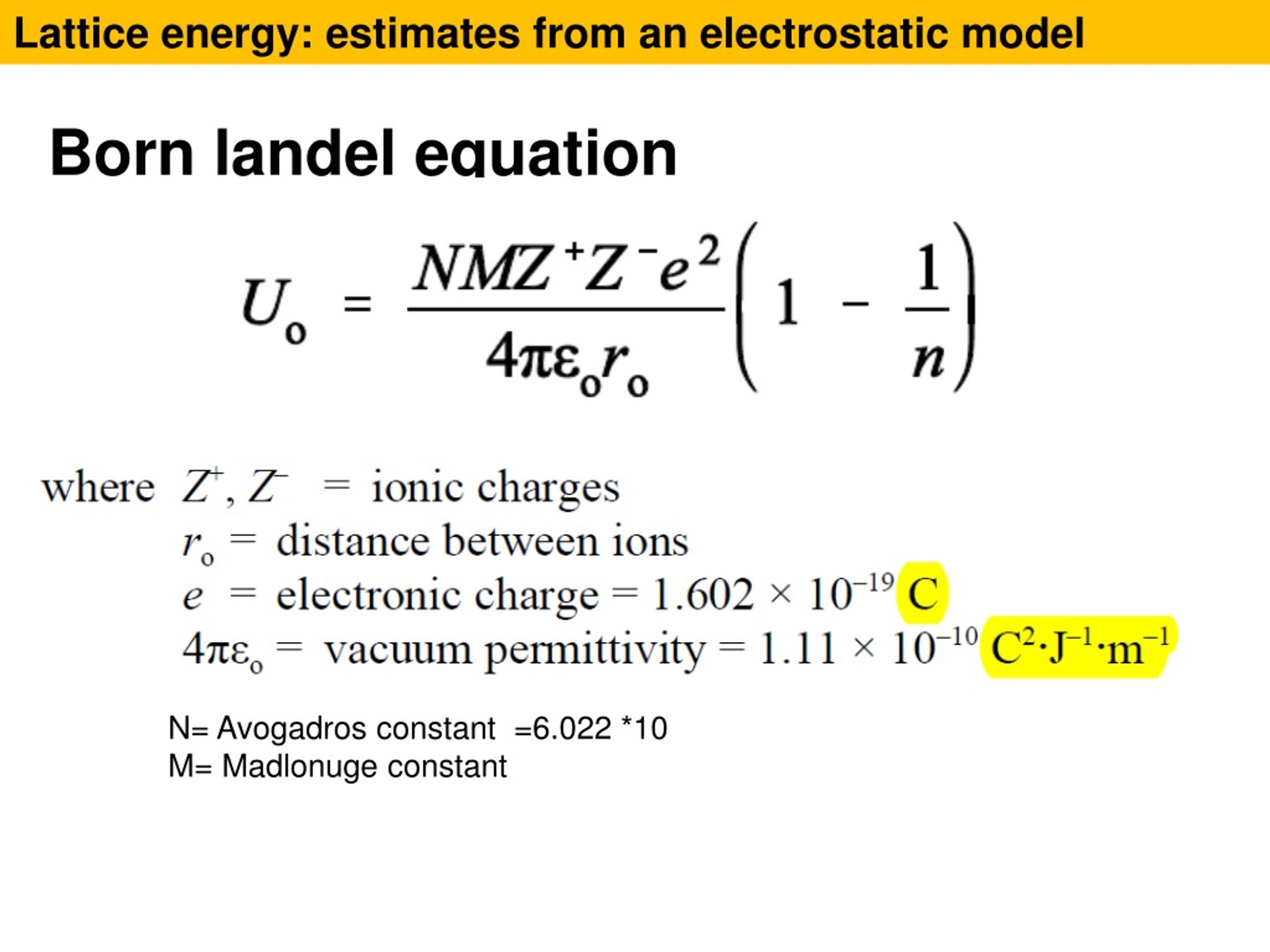
If you know how to do it, you can then fairly easily convert between the two. Calculations of this sort end up with values of lattice energy, and not lattice enthalpy. By doing physics-style calculations, it is possible to calculate a theoretical value for what you would expect the lattice energy to be. Let's also assume that the ions are point charges - in other words that the charge is concentrated at the center of the ion. Let's assume that a compound is fully ionic. Theoretical Estimates of Lattice Energies Once again, the cycle sorts out the sign of the lattice enthalpy. This time both routes would start from the elements in their standard states, and finish at the gaseous ions. You cannot use the original one, because that would go against the flow of the lattice enthalpy arrow. It does, of course, mean that you have to find two new routes. The only difference in the diagram is the direction the lattice enthalpy arrow is pointing. How would this be different if you had drawn a lattice dissociation enthalpy in your diagram? Your diagram would now look like this: So, from the cycle we get the calculations directly underneath it. The diagram is set up to provide two different routes between the thick lines. Now we can use Hess' Law and find two different routes around the diagram which we can equate. And finally, we have the positive and negative gaseous ions that we can convert into the solid sodium chloride using the lattice formation enthalpy.Remember that first electron affinities go from gaseous atoms to gaseous singly charged negative ions. The -349 is the first electron affinity of chlorine.Again, we have to produce gaseous atoms so that we can use the next stage in the cycle. The +122 is the atomization enthalpy of chlorine.
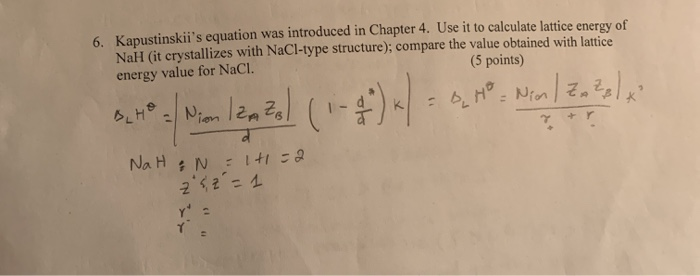
Remember that first ionization energies go from gaseous atoms to gaseous singly charged positive ions.
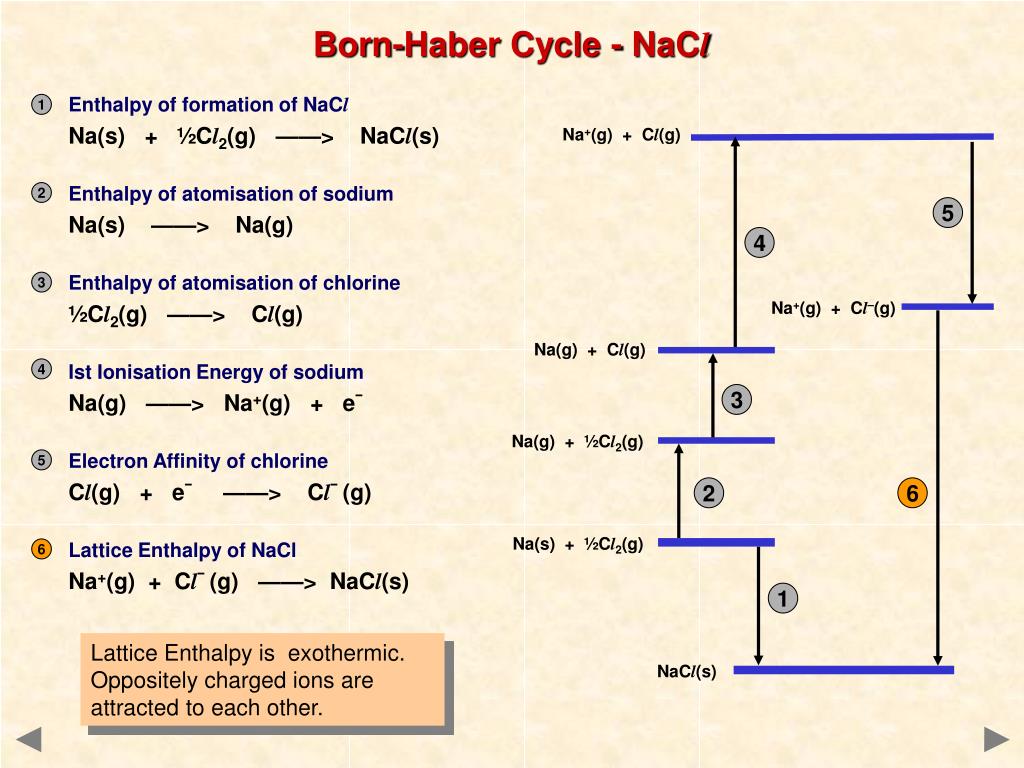
If you wanted to draw it for lattice dissociation enthalpy, the red arrow would be reversed - pointing upwards.įocus to start with on the higher of the two thicker horizontal lines. You will see that I have arbitrarily decided to draw this for lattice formation enthalpy. \)Ĭonsider a Born-Haber cycle for sodium chloride, and then talk it through carefully afterwards.


 0 kommentar(er)
0 kommentar(er)
